We’re recruiting. If you’re interested in learning how the brain responds to abiotic stressors, see these projects or bring your own ideas to matthew.meiselman@unlv.edu
The molecular and neural substrates for insect dormancy
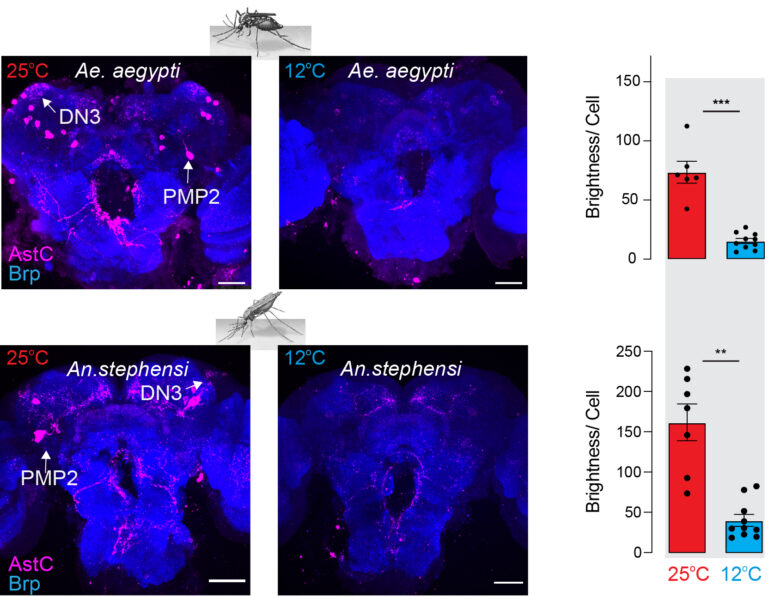
Mosquitoes depend upon dormancy to bridge survival through winters in the north and dry seasons in the equatorial regions. Our current understanding of the brain circuitry connecting linking environmental perception to metabolic arrest is poor. The circadian system has been implicated in dormancy in many species of mosquitoes, but no neurons or signaling molecules by which the circadian system conveys its consummate assessment of the environment have been found. To efficiently uncover the molecular and neural substrates involved in execution of mosquito dormancy, I will leverage the rapid progress possible in D. melanogaster, which is easier to rear and far more genetically accessible.Matthew’s recent work showed that circadian neurons (DN3s) in the fly brain are temperature sensitive, as is expression of their operant neuropeptide AstC. Cold inhibits AstC release leading to activation of cholinergic neurons expressing the Gi/o coupling AstCR2. Activation of AstCR2 neurons induces reproductive dormancy in warm. This represents an excellent access point to elucidate the core circuitry of dormancy, which I expect to share common elements in flies and mosquitoes.or
Ixodes scapularis females exit a dormant state when temperatures drop below 10C
Ethological responses to temperature change
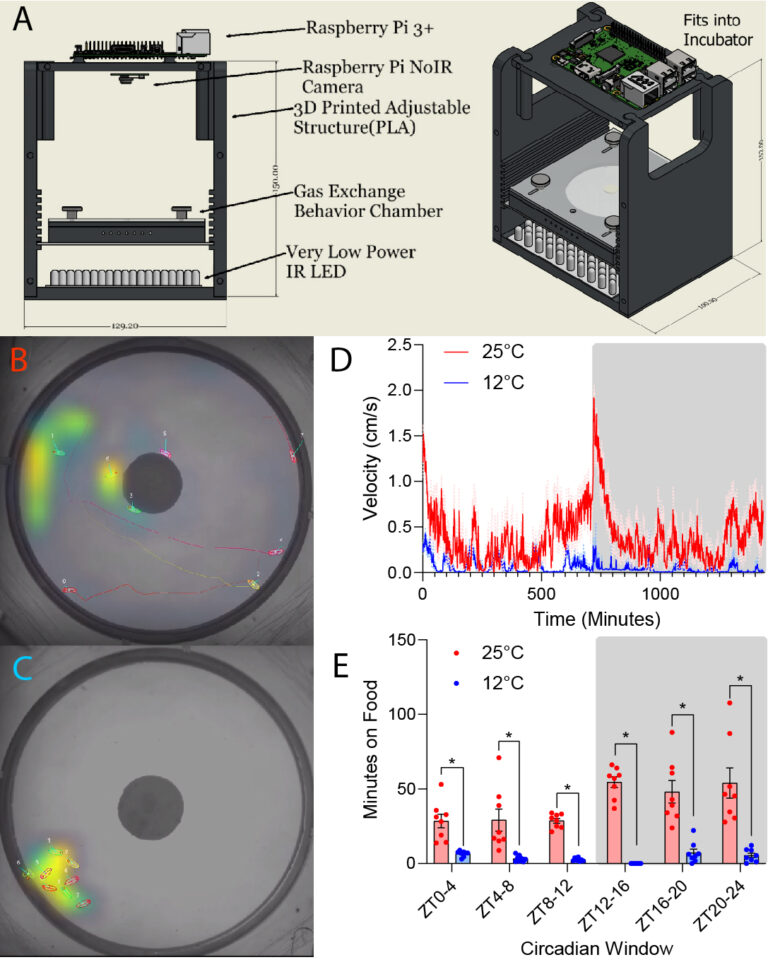
Predatory disease vectors have very specific conditions in which they will host seek. When the temperature deviates from those conditions, organisms must rapidly adapt their behavior, and in many cases, seek shelter from the elements. We have collected preliminary data in flies, mosquitoes and ticks that show dramatic changes to overall activity, structure of circadian rhythmicity, and phototaxis. We are using our recently developed overhead camera (LOOC) for recording and T-Rex software for multi-animal tracking.
Modulation of reproduction
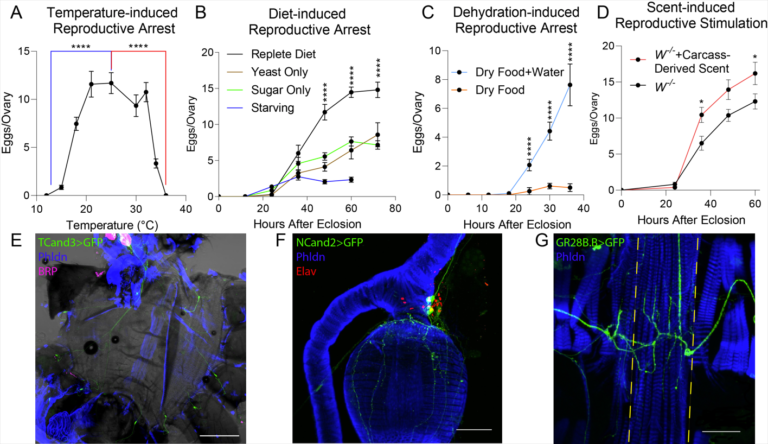
Organisms must choose the optimal time and environment to reproduce, not only because a poor decision can lead to costly misallocation of resources, but also in order to put their offspring in a position to thrive. We found cold, heat, nutritional stress, dehydration, and the presence of carcass derived scent, all modulate egg production. Via unbiased screens, I identified 27 neuron subsets that either stimulate or inhibit egg production. These subsets are mostly non-overlapping and label diverse neurons, including abdominal body wall neurons, enteric neurons, and pericardial neurons. We will create highly specific drivers for functional characterization. Aided by the full adult female brain (FAFB) electron microscopy dataset, functional imaging, and neural tracing techniques, we aim to map each circuit for integration with known food/water/heat sensing paths, or identification of novel ones. The unbiased approach that identified these neurons may lead to novel molecular mechanisms for interoception and exteroception, as well as convergence hubs which coordinate stressors with reproduction. We’re hoping to find new receptors influencing fecundity, which could be excellent targets for vector population control.